Liraglutide and Weight Loss - The Real Skinny
/For the video version of this post, click here. Weight loss is something of a holy grail for pharmaceutical companies. A large and, frankly, growing market exists not only in the US but around the world, and is only getting bigger. The list of drugs that have tried, and failed, to crack this market is ever-growing.
Weve recently seen a slew of news reports about the SCALE study touting the injectable medication liraglutide, now being marketed by Novo Nordisk as Saxenda.
The mainstream news outlets have done their job getting the details of this trial out there,
but when your patients ask you about liraglutide, you might want a little more detail than what you get from CNN. So, this week, were taking a second look at the SCALE study.
Lets start at the beginning.
Liraglutide is a glucagon-like-peptide 1 analogue, and, as a peptide, can only be given by injection. GLP-1 is in the incretin family - it increases insulin and insulin sensitivity, delays gastric emptying, and promotes feelings of satiety. As such, liraglutide was originally tested in, and approved for use in, individuals with type 2 diabetes. That weight loss was a side-effect was I think a happy accident for Novo Nordisk.
The trial, as its presented, was pretty straight-forward. We have roughly 3700 non-diabetic patients with a BMI greater than 30 or 27 if they had a relevant comorbidity randomized to liraglutide or placebo and followed for 56 weeks.
They also got lifestyle intervention. The endpoint was change in weight, and it was pretty clear that the liraglutide group lost more than the placebo group - around 8 kilograms versus 3 kilograms.
The news outlets have, appropriately, pointed out that for 5 kilograms of weight over a year, the $1000 a month price tag might not be reasonable, but we can dig a bit deeper than that.
Lets talk study design. First of all, check out this sentence regarding statistical power.
This study was designed with a whopping 99% power to detect a positive outcome. Actually, by my calculation, it was around 99.99% power. If Novo Nordisk was going for a more traditional 90% power, they would only have had to recruit around 150 individuals. In other words, they were betting BIG on this drug. Whether it paid off is still up for grabs.
Heres another point worth noting - statistical analysis and editorial assistance for the manuscript were provided by Novo Nordisk. Given that most of the endpoints were pre-specified, they didnt have too much flexibility to massage the data, but I think it would be informative to consider what doesnt appear in the manuscript.
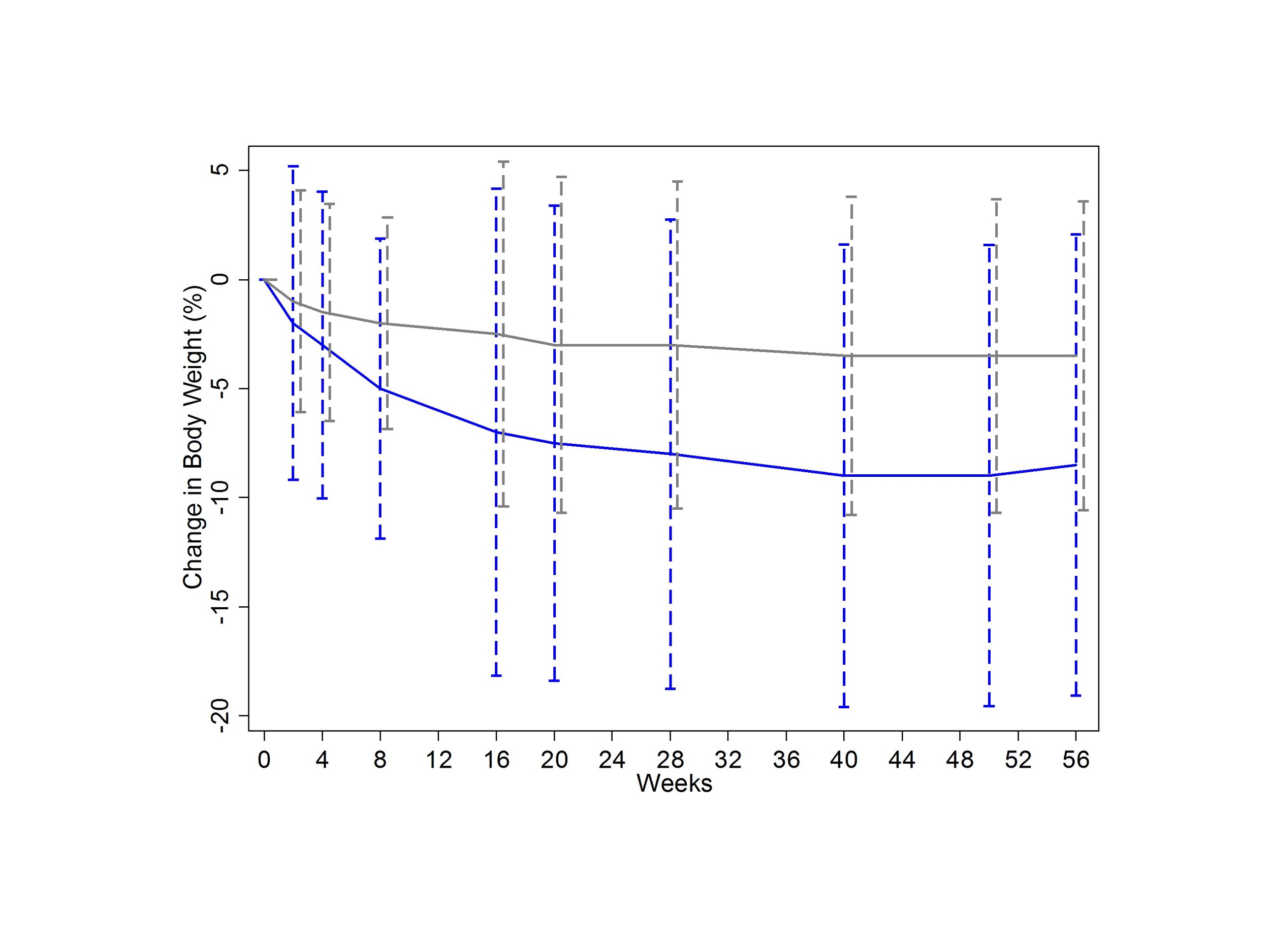
Take a look at Figure 1 here.
This is the important figure - weight-loss in the two comparison groups. How would you interpret the error bars? If they are standard deviations, the graph would suggest that weight loss was remarkably consistent in the liraglutide group. But those error bars arent standard deviations, they are standard errors - a subtle difference, but one with a big impact. Heres what the graph would look like with standard deviations.
This would help to drive home the fact that, although the drug seems to work, your mileage may vary.
Theres also no discussion of how well blinding worked. Did people who got placebo know they were getting placebo? Reading between the lines, it seems pretty clear that they did. 20% of the placebo group withdrew their consent for the study, only 10% of the liraglutide group did.
They could probably figure it out because the side-effects of liraglutide were so clear. 25% of liraglutide patients had nausea in the first four weeks, as this graph shows.
One other analysis I would have liked to see? Adjust the outcome for the side-effect symptomatology. In other words, do people lose weight because they feel sick, or is that just an unlucky effect that occurs in some. It wouldnt invalidate the results, certainly, but it would certainly help us counsel patients.
Now, this trial was conducted in 27 countries at 191 different sites. The company would state that they did this to increase generalizability. There are some more cynical reasons to do this though. Number one, its a lot cheaper to do these studies outside of the US. But research oversight can also be a bit more lax.. in other countries. Despite my searching, I was unable to turn up how many of these patients came from the US. In a study where blinding was likely incomplete, it would be very interesting to see if certain recruitment sites had spuriously high effect sizes.
Now let me be clear, Im not saying anything untoward happened here, only that the data would be nice to have.
As for the main issue you see in the press? That the drug is too expensive for its modest efficacy? Lets not throw the baby out with the bathwater. One interesting secondary outcome was the rate of new diabetes diagnoses - low overall, but 7-fold higher in the placebo group.
One unfortunate, though unsurprising, finding was that the weight comes back when you stop using the drug, as evidenced from an extension trial in the liraglutide arm. So this might be a life-long drug. Good for Novo Nordisk, but bad for patients.
After a second look, where are we left with liraglutide? Well, the drug causes weight loss, in most people. It causes side-effects, in a lot of people. It may prevent diabetes. It costs a lot. The rate of uptake is going to be heavily dependent on calculations done by the insurance companies. If the co-morbid conditions ameliorated or prevented by the weight loss will save money, well see it get used. But its not a permanent fix. Theres an elephant in the room that isnt mentioned in the manuscript, and in few of the news articles I read. Its called bariatric surgery, a one-time procedure which may have more efficacy than a lifetime of liraglutide. And though that wasnt the comparator in this trial, it may be the comparator in our patients heads.
Thats the skinny on liraglutide. Ill see you next time we need to take a second look.